I discussed the importance of organoid research with one of the pioneers in the field, Hans Clevers of the Hubrecht Institute in the Netherlands. His edited responses are in italic.
How can these ‘mini-organs’ combat the pandemic?
Human and animal organoids are proving their value because you can infect them with SARS-CoV-2, which is transmitted through the air like other highly-contagious viruses, to help understand its biology, from how they infect the lung to other organs.
Organoids are as close as scientists can get to seeing the effects of a disease on tissues outside of studying people themselves and provide a useful halfway house between studies of individual cells and studies of people.
This approach was used to show how Zika virus caused microcephaly, a birth defect in which a baby’s head is smaller than expected, for example.
In the case of COVID-19, colleagues around the world have used them to see how the virus infects tissues identify promising drugs and have even made organoids based on the tissues of bats, the likely source of the virus, to understand how the virus jumps between species.
How do you make an organoid?
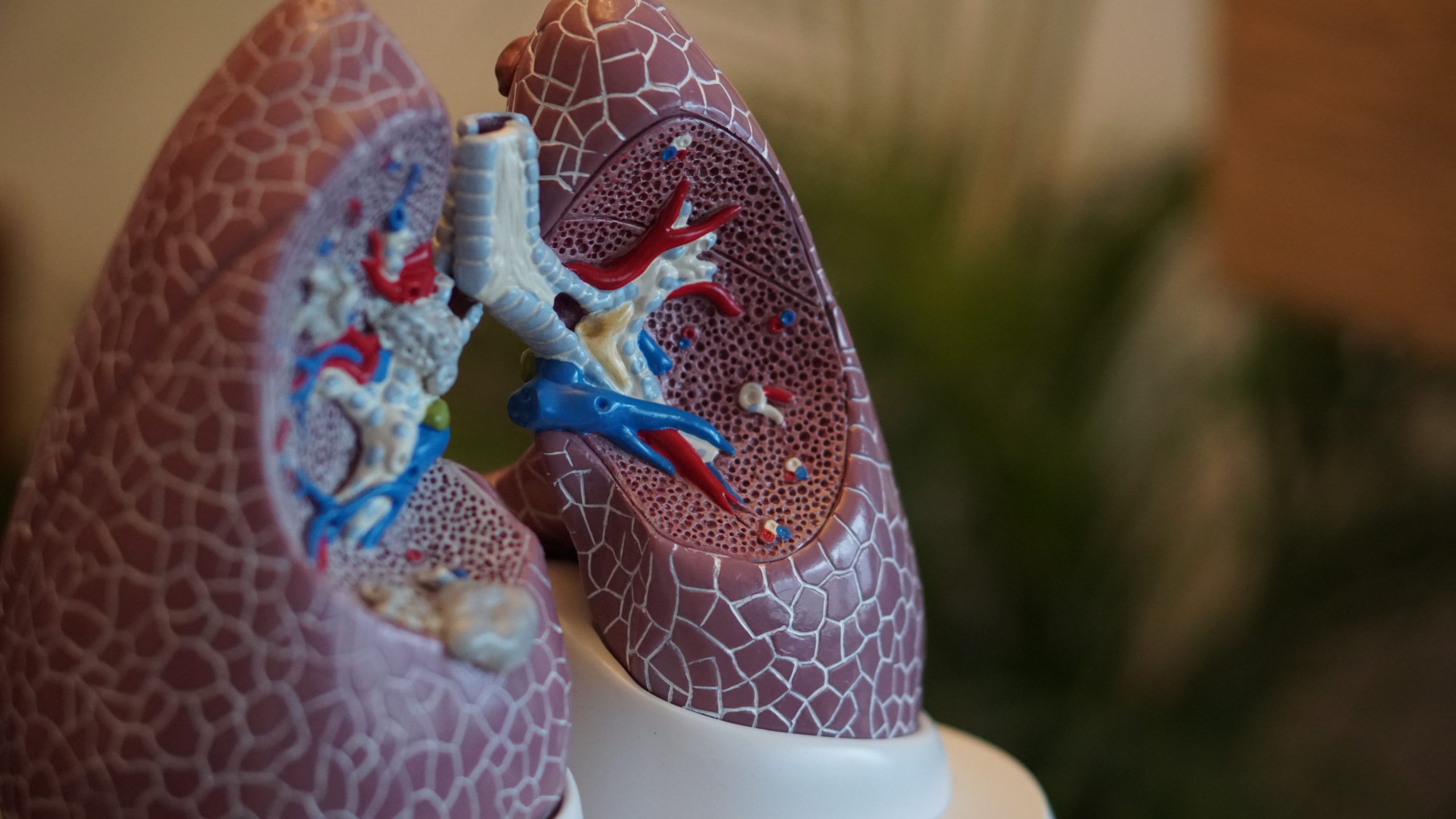
There are two organoid technologies, both of which arose just over ten years ago, that you can use to make real copies of real organs.
They both start from stem cells, which are ‘parents’ of other cell types in the body, which can be used – if you mimic the right conditions – to build-up an organ.

In one approach, you take a cell from an early embryo, a so-called embryonic stem cell or, more easily, take an adult cell, like a skin cell, and make it embryonic again by genetic modification (the latter are called induced pluripotent stem cells, or iPS cells, and their development in 2006 led to a 2012 Nobel prize for Shinya Yamanaka).
The hard part is finding ways to persuade these stem cells to differentiate, that is to turn into other cell types, to make three-dimensional organoids.
You first grow them into a clump, called an embryoid body, then you take them through the normal steps of development. In effect, you keep on restricting what they can become, and you drive them towards becoming a brain, kidney, lung or whatever.
You have to find ways to mimic the complicated signals – such as growth factors – that are made in the body during normal development. These are complex protocols and, in the case of the human brain, it can take nine months to make a tiny part.
You can also isolate stem cells from adult tissues, which is what my team does.
We have many types of adults stem cells that arise during the development of an organ. They maintain the organs in the body and when they become overactive, you get cancer, while ageing is linked to them being underactive – you can think of ageing as a stem cell disease.
Our technology starts from a little piece of gut, skin, lung, or liver. We dip them into a gel, called Matrigel, which mimics the normal environment of cells, and give them the correct set of growth factors.
With the right balance and times, the stem cells behave as if they are maintaining the tissue. So, if you take the stem cells from guts, for example, you get fantastic mini guts that grow forever.
How big can you grow a mini-organ?
When it comes to using iPS or embryonic stem cells, there is very little increase of mass after the creation of the embryoid body. But when you grow organoids from adult stem cells they keep on cranking out cells and can grow for years.
But both are limited by the amount of oxygen and nutrients you can get to the cells within the organoid – you need blood vessels, a pumping heart and lungs connected to oxygenate the blood. Bioengineers want to create different organoids on a chip to do this, but it is more dream than reality.
Take gut organoids, for example. Like the real gut, they are hollow, and we end up with a balloon of cells that can grow up to a millimetre or so, though there is no real limit. They are visible to the naked eye as white dots.
For solid organs, such as mini-brains and mini-kidneys made from iPS cells, you can grow them one or two millimetres, but beyond that, cells in the centre start dying off because they don’t get enough oxygen and nutrients.
Even so, it is surprising the extent to which they can self-organise to form structures seen in a real body.
Does the small size of organoids matter?
You can recapitulate the essential features of a gut, liver, lung or whatever in tiny organoids because organs often consist of highly-repetitive building blocks.
The liver, for example, contains a million structures called lobules, which are essentially the same. If you grow ten, you don’t gain much more by growing ten million.
In the intestine, there are little cavities called crypts and tiny projections called villi, which only vary a little across the gut. But you don’t have blood vessels and nerves. The reason is that there are three basic cell types;
- The gut organoids are grown from what are called endoderm cells.
- The nervous system organoids come from the ectoderm type.
- The circulatory system organoids come from the mesoderm type.
You can also grow human airway organoids lined with four key cell types – ciliated cells, goblet cells, club cells and basal cells – and structures, such as the air sacs in lung tissue.
However, the brain is immensely more complex than any other organ. Every region only occurs once, on each side, and thousands of different functions are each supported by their own unique region.
When people talk about ‘mini-brains’, you have to remember that it is hard to control what you make, whether brain stem or cortex.

How do organoids change virology?
Whenever a new infectious disease emerges, virologists expose cell lines — typically cancer cells from a monkey or human origin— to swabs and other samples from patients and then search for evidence of replicating virus. Virologists often use ‘Vero cells’, a green monkey kidney cell, for example.
But this does not model the SARS-CoV-2 infection well because cell lines do not mimic what happens in the body: if you transplant these cell lines into the body, you get a tumour, while if you transplant an organoid, they contribute to an organ’s tissue.
This is also a trial-and-error approach because the human body contains many hundreds of different cell types and some are more susceptible than others.
Mary Estes and colleagues in Texas were the first to show the potential of organoids in virology in their studies of noroviruses, which cause vomiting and acute gastroenteritis. Until their work, these viruses could not be grown in the laboratory with Vero cells or any other cell line for that matter.
Using intestinal organoids, they found the virus infects gut enterocytes, a cell type that’s absent from intestinal cell lines yet is present in intestinal organoids.

In the case of this virus, a protein spike on its surface binds to human cells through a docking point, called a receptor, in this case one called ACE2 that responds to a human hormone called angiotensin. Some cells have more ACE2 receptors than others.
How does the coronavirus affect lung tissue?
Kazuo Takayama at Kyoto University, Japan, and his team have developed bronchial organoids with four cell types and found that the virus mainly targets the stem cells that replenish cells in the lining, known as basal cells.
What can organoids tell us about how the coronavirus spreads in the body?
SARS-CoV-2 causes Influenza-like symptoms ranging from mild disease to severe lung injury, yet it affects other organs, notably the gastrointestinal tract and the kidney. Until organoids were used, it was not clear whether this damage is directly caused by the virus or a complication caused by the response of the body to the infection.
Three teams – including my own – used human intestinal organoids to examine whether the new coronavirus could establish itself in the gastrointestinal tract.
This is because cells there have high levels of the ACE2 receptor used by the virus to enter cells and the viral genetic material is regularly found in anal swabs, stool and sewers (the latter can be used to monitor what is going on in a population).
All three studies reported that the most common cell type of the intestinal epithelium (lining), the enterocyte, is readily infected, suggesting that the intestine is a potential site of SARS-CoV-2 replication.
Among other things, these enterocytes contained certain enzymes called proteases (TMPRSS2 and TMPRSS4) which helped the virus to invade cells.
Although the stomach is acidic and contains digestive enzymes, we think the COVID-19 virus can be protected by dead cells and mucus to go from the respiratory tract to the gut.
We found the virus infected the gut frighteningly well. My son, who is working in a hospital ward, was struck by how many COVID-19 patients had stomachache, nausea and diarrhoea before the respiratory disease.
A team co-led by Joseph Penninger of the University of British Columbia and Institute of Molecular Biotechnology, Vienna used blood vessel organoids to show that SARS-CoV-2 can infect the endothelium — the cells lining the blood vessels — to circulate around the body.
The virus is linked with coagulation, blood clotting, which might be a major factor in the rapid decline and death in older and susceptible patients.
It is not understood how the virus does this but it is probably linked with the changes it causes in endothelial cells, in blood vessel walls to cause coagulation, and the resulting clots can move around the body, for instance to the brain.
The virus could also directly infect kidney organoids. This may explain the loss of kidney function in severely ill individuals.
Another study in liver organoids at Fudan University, Shanghai, found that the virus can infect and kill cells that contribute to bile production, known as cholangiocytes.
Before that, researchers had suspected that the liver damage seen in people with COVID-19 was caused by an overactive immune response or drug side effects.
Finally, some reports indicate that 30-60% of patients with COVID-19 suffer from infection of the central nervous system and various studies suggest that the virus can infect brain organoids. If it crosses the blood-brain barrier, which is not easy for a virus, it could infect brain tissue.
An £8.4 million research study into the long-term health impacts of COVID-19 on 10,000 hospitalised patients has been launched in Leicester, while a major online survey of how COVID-19 affects cognition is underway by Adrian Owen, at the University of Western Ontario:
“As people are starting to recover from COVID-19 we are seeing more and more of them having to cope with cognitive challenges like problems with memory, concentrating and problem-solving” said Owen.
“We want to find out whether these problems are caused by direct viral effects on the brain itself or indirect effects due to inflammation, blood clots, low oxygen levels, sedation and spending time on a ventilator.”
Can you test potential COVID-19 drugs on organoids?
Yes, organoids can be used to test potential COVID-19 therapies.
We have already done to find which cystic fibrosis patients will respond to an expensive new drug, an approach that is also being adopted in cancer therapies to grow a tumour organoid from a patient’s tissue to see how they will respond to drugs in the clinic. It is unbelievably predictive but it is expensive, slow and can take weeks.
In the case of COVID-19, Shuibin Chen of Weill Cornell Medicine, New York, screened some 1,200 drugs and found that the cancer medication imatinib and mycophenolic acid suppressed SARS-CoV-2 in lung organoids.
In human bronchial organoids, treatment with the drug camostat reduced the amount of virus, according to a study by Kazuo Takayama of Kyoto University and colleagues.
Meanwhile, Jan Münch of the Ulm University Medical Centre, Germany, and colleagues showed that Remdesivir, one of the more promising drugs to treat COVID-19, suppressed SARS-CoV-2 infection in intestinal organoids.
How else can organoids be used to treat COVID-19?
What I hear from clinics and patient organisations is that the lung damage caused by coronavirus is striking, causing long term respiratory problems, unlike other viruses.
We are part of a big international consortium, including Paolo de Coppi at Great Ormond Street Hospital to see if we can use stem cells, including in the form of organoids, for instance of structures in the lung, called alveoli.
Hans Snoeck’s lab at the Columbia University Medical Center in New York City, has grown lung organoids which, when inhaled by mice with lung damage, did re-establish working tissue. Of course, this is only on mice and, for a transplant, you would need a lot of mini organs.
What about bat organoids?
In the wake of the SARS epidemic, numerous related coronaviruses were identified in faecal samples or anal swabs of Chinese horseshoe bats, but we did not find ways to grow them in the laboratory using bat cells.
Based on the similarity of SARS-CoV-2 to coronaviruses in horseshoe bats, Kwok Yung Yuen of The University of Hong Kong, Hong Kong, China, and colleagues have made intestinal organoids from the horseshoe bat species Rhinolophus sinicus.
These are readily infected by SARS-CoV-2 and it will be interesting to use the bat organoids to reinvestigate these viruses to better understand them.

The image was captured and colorized at NIAID’s Rocky Mountain Laboratories (RML) in Hamilton, Montana.
Are other species of interest for the creation of organoids?
The armamentarium of virology may need to be expanded with airway and intestinal organoids derived from other species infected by coronaviruses, such as civets and pangolins. Such an approach will minimize the impact of coronavirus research on these endangered species and may prepare us better for a next pandemic.
What are the limits of what you can do with organoids?
More complex organoids will be needed to understand how the SARS-CoV-2 virus interacts with the body’s immune system.
In general, studies of the virus in organoids do not reflect the interaction between organs that happens in the body, which means that key findings will still need to be validated in studies of animals and patients.
HOW CAN I FIND OUT MORE?
The latest picture of how far the pandemic has spread can be seen on the Johns Hopkins Coronavirus Resource Center or Robert Koch-Institute.
You can check the number of UK COVID-19 lab-confirmed cases and deaths along with figures from the Office of National Statistics.
There is more information in my earlier blog posts (including in German by focusTerra, ETH Zürich, with additional information on Switzerland), from the UKRI, the EU, US Centers for Disease Control, WHO, on this COVID-19 portal and Our World in Data.
The Science Museum Group is collecting objects and ephemera to document this health emergency for future generations.